Surface tension
The surface tension in soap bubbles is increased by the water and decreased by the soap

You can make floating soap bubbles in many ways. Some of them are presented here in three categories: Ways dealing with weight, ways dealing with air pressure and ways dealing with electricity.
A soap bubble will float if it is filled with helium or some other light gas. The bubble will float just like balloons filled with helium because its total weight is smaller than the same volume of air. If you have a flask of helium it is really easy to make the floating bubbles. All you need to do is to dip a hose that is connected to the gas flask into your bubble mixture and gently open for the gas. If you are using hydrogen instead you can make beautiful explosions by setting fire to the bubbles. Most people, however don’t have access to gas flasks (and maybe that’s a good thing) and if you have the access, remember to only carry out the experiment together with a person who is educated in handling gas flasks. If you do not have access to hydrogen or helium you can for example see the experiment done at soap bubbles show in Experimentarium, the science centre in Copenhagen.
Instead of filling the bubbles with a light gas, you can fill its surroundings with a heavy gas. The weight of soap bubbles is not much higher than the weight of air because the soap film in the bubbles is incredibly thin. If the soap bubbles are made in a container with a gas heavier than air, the bubbles will float on the heavy gas. An example of a heavy gas is CO2. Frozen CO2 is called dry ice. At room temperature the ice sublimates’ into gas (It doesn’t melt into a liquid phase, and this is why we call it dry ice). If you put dry ice into a big container and blow bubbles into it, they will float on the CO2. If you don’t have access dry ice, you can make the CO2 yourself using baking soda and vinegar. Pour both parts into the container (start with the vinegar) and CO2 will quickly fill the container. By the way, the produced CO2 is exactly the same as would have made your bread swell if you had used the baking powder for making a bread instead.
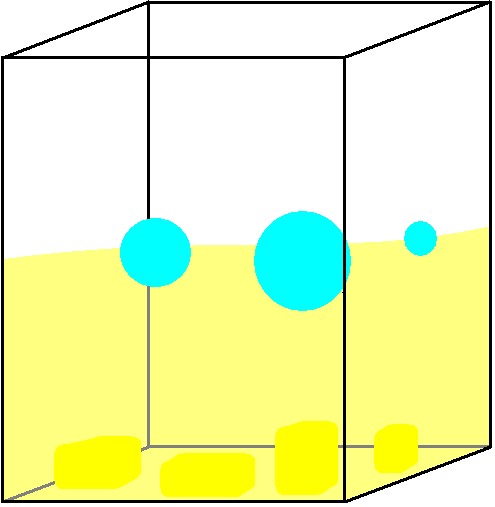
CO2 gas is produced from vinegar and baking soda. A bubble is put into the glass container. Bubbles in the container will float on the gas. The CO2 is invisible so it will appear as if the bubbles were floating in air.
Another strategy is to make hot air balloons out of the soap bubbles by filling them with hot air. A litre of hot air has a lower weight than a litre of cold air, so if the air in the bubble is sufficiently hotter than the air outside, the bubble will float. In cold weather one can use body heat to make hot air bubbles by filling the bubbles with hot air from the lungs. Use a funnel (e.g. made from paper) to make sure that all the air in the bubble comes from the lungs only and to be able to make big bubbles quickly. It is a nice experiment, but it has to be carried out in rather cold weather if it should work properly. When we have performed the experiment it has not always worked. You can try to produce hot air in other way, but results from our experiments with e.g. hair dryers has until now not turned out well.

Experimental setup for making bubbles filled with steam. The hot steam will make the bubble rise until the bubble has cooled sufficiently and the bubble will fall again.
An easy way to make the bubble float is to induce movements in the air above the bubble. You can use a hand or a little piece of paper. When the air above the bubble is moving the air pressure in this region will fall. The pressure will be lower in a region with moving air relative to a region with slower movements. The principle is described mathematically by Bernoulli’s equation,
where is the air pressure, is the density of the air, is 9.8 m/s2, is the height above a chosen zero point, and is the velocity of the air. For a small bubble the height across the bubble () is negligible and if the air under the bubble is not moving (), then we can reduce the Bernoulli equation to:
We can therefore realize that the air pressure above the bubble will fall if the air in this region is moving. The bubble will float because it is sucked upwards to the region with lower pressure. Air planes are flying using the same principle. A wing is designed so that the air is moving faster above the wing than under it. Therefore the wing – and the plane – will be sucked upwards. The principle of a wing can therefore be illustrated using soap bubbles.
Soap bubbles can be attracted by electrical fields. Therefore it is possible to make soap bubbles float using static electricity. Charge a balloon for example by rubbing it against your hair, and make a small soap bubble right under the balloon. The balloon will now attract the bubble. If you carefully adjust the distance between the balloon and bubble, you can hold the bubble at the same height for minutes.

The surface tension in soap bubbles is increased by the water and decreased by the soap
The bubble is attracted because of the polar forces in the water. In an electric field all water molecules will be aligned with their positive pole in direction towards any negative charges nearby. A charged balloon will be negatively charged. So the water molecules will align their positive poles toward the balloon and their negative poles away from it. The negative pole will be repelled from the balloon, and the positive will be attracted. Because the positive poles are closer to the balloon than the negative, the resulting force will be attractive.
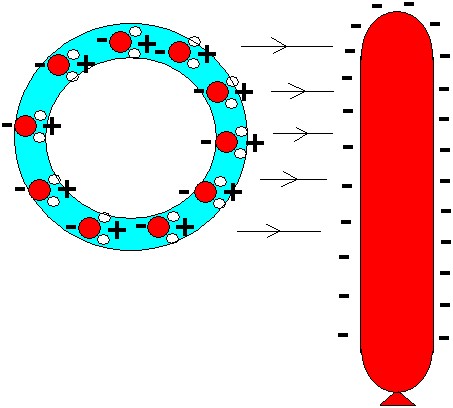
A charged balloon will attract the water molecules in a soap bubble. The polar water molecules will be attracted by the negatively charged balloon. Therefore the balloon will attract the bubble.
If both the balloon and a bubble are charged negatively, they will repel each other. This is possible to achieve with soap bubbles too. The soap bubbles can then float above the balloon.